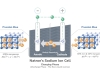
The batteries use aluminum and sulfur as its two electrode materials, sandwiching a molten salt electrolyte and have been described in the popular science magazine Nature.
MIT Professor Donald Sadoway said the battery cells could endure hundreds of cycles at exceptionally high charging rates, with a projected cost per cell of about one-sixth that of comparable lithium-ion cells. They showed that the charging rate was highly dependent on the working temperature, with 110 degrees Celsius (230 degrees Fahrenheit) showing 25 times faster rates than 25 C (77 F).
Surprisingly, the molten salt the team chose as an electrolyte simply because of its low melting point turned out to have a fortuitous advantage.
What's more, the battery requires no external heat source to maintain its operating temperature. The heat is naturally produced electrochemically by the charging and discharging of the battery. "As you charge, you generate heat, and that keeps the salt from freezing. And then, when you discharge, it also generates heat," Sadoway said.
In a typical installation used for load-leveling at a solar generation facility, for example, "you'd store electricity when the sun is shining, and then you'd draw electricity after dark, and you'd do this every day. And that charge-idle-discharge-idle is enough to generate enough heat to keep the thing at temperature."
This new battery formulation, he says, would be ideal for installations of about the size needed to power a single home or small to medium business, producing on the order of a few tens of kilowatt-hours of storage capacity.
For larger installations, up to utility scale of tens to hundreds of megawatt hours, other technologies might be more effective, including the liquid metal batteries Sadoway and his students developed several years ago and which formed the basis for a spinoff company called Ambri, which hopes to deliver its first products within the next year.
The smaller scale of the aluminum-sulfur batteries would also make them practical for uses such as electric vehicle charging stations, Sadoway says. He points out that when electric vehicles become common enough on the roads that several cars want to charge up at once, as happens today with gasoline fuel pumps, "if you try to do that with batteries and you want rapid charging, the amperages are just so high that we don't have that amount of amperage in the line that feeds the facility."
So having a battery system such as this to store power and then release it quickly when needed could eliminate the need for installing expensive new power lines to serve these chargers.
"The first order of business for the company is to demonstrate that it works at scale," Sadoway says, and then subject it to a series of stress tests, including running through hundreds of charging cycles.